PARK CITY, UTAH, USA, June 28, 2022 /EINPresswire.com/ — Warning letters issued by the FDA to manufacturers and/or distributors of devices marketed for regrowing hair, weight loss, spider vein removal, and dermabrasion, as well as injectable fillers and decorative contact lenses, illustrate an important legal distinction – the differences between the legal definitions of cosmetics and medical devices. Although the devices referenced in these warning letters are designed to change a person’s appearance, their intent to diagnose or treat a medical condition or influence the structure or function of the body classifies them as medical devices under the Federal Food, Drug, and Cosmetic Act (FD&C Act).
The Food and Drug Administration (FDA) controls the following goods in general: Biologics; Cosmetics; Some Dietary supplements; Food additives; Infant formulas; Medical devices (the FDA classifies them as class I, II, or III); Over-the-counter pharmaceuticals; Prescription drugs; Radiation-emitting items; Surgical implants; and Tobacco products.
It is essential to grasp the distinction between FDA “approved” and FDA “cleared” when it comes to lawsuits involving faulty medical devices, particularly as more consumer electronics products reach the market. Because there is a substantial contrast between approved and cleared: While FDA-approved means that the FDA has determined that the benefits of the product outweigh the known risks and manufacturers must submit a premarket approval application and clinical testing results to be approved, FDA-cleared means that the manufacturer can demonstrate that the product is “substantially equivalent” to another similar legally marketed device that has already been approved (i.e., a “predicate”).
Medical Devices classifications
The FDA classifies medical devices into three categories: Class I (least risk), Class II, and Class III (highest risk). The following are instances:
Class I: Bandages, enema kits, etc. Approximately 93% of these products are exempt from premarket review.
Class II includes motorized wheelchairs, pregnancy tests, etc. Most need notice before commercialization under 510(k);
Class III: breast implants, pacemakers, and so on. Require premarket clearance or humanitarian device exemption.
If a device is deemed “exempt,” the maker is exempt from submitting a premarket notice submission (510(k)) to gain approval before marketing the device. Thermometers containing mercury, heating pads, bedpans, etc., are a few examples. Still, producers must register with the FDA and list their products.
When it comes to “cleared” devices, producers file a premarket notice submission to the FDA, and after the agency determines that it is substantially comparable to a predicate, the product is cleared and may be marketed. However, corporations are occasionally accused of obtaining approved goods by employing items previously recalled for safety concerns; thus, FDA clearance does not provide automatic protection against a lawsuit. Additionally, the FDA does not perform its testing; instead of relying on the findings of tests conducted by other independent laboratories.
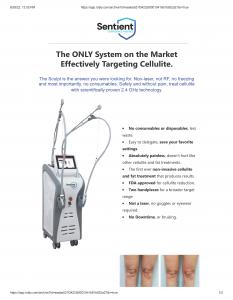
“Several companies, such as Sentient lasers, misrepresent the FDA clearance as FDA approval,” Said Dr. Alhallak, the director of Albany Cosmetic and Laser Center. “For example, Sentient Lasers website shows that Sentient Sculpt TM is FDA cleared for cellulite treatment in all skin types, but their social media accounts show that the device is FDA approved. However, I could not find any approval or clearance for the device in the FDA medical device database.” He ended.
Apart from misrepresenting the facts, such practice might put practitioners at risk of malpractice liability if they used a non-cleared device!
—
NOTICE: This e-mail contains information that may be confidential. If you are not the intended recipient, any disclosure or other use of this e-mail or the information contained herein or attached hereto may be unlawful and is strictly prohibited. If you have received this e-mail in error, please notify the sender immediately and delete this e-mail without reading, printing, copying or forwarding it to anyone. Thank you for your kind collaboration.
Dr. Kamal alhallak
Albany Cosmetic and Laser Centre
+1 587-520-2835
email us here
![]()
Article originally published on www.einpresswire.com as FDA issued a warning Letters Highlight Differences Between Cosmetics and Medical Devices

 ,
,
